Name of the Organization : Food & Drug Administration
Type of Facility : DocTrack Status
Country : Philippines
Website : http://www.fda.gov.ph/
How To Do FDA DocTrack Status Tracking Philippines?
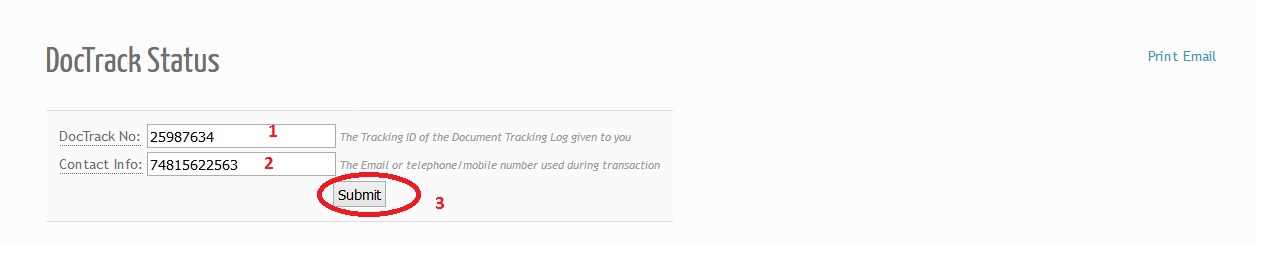
Visit FDA Philippines website for DocTrack Status Tracking. The Tracking ID of the Document Tracking Log given to you
Related / Similar Service : DTI BN Registration Philippines
Contact Info:
The Email or telephone/mobile number used during transaction
Mandate of FDA
As a regulatory agency under the Department of Health, the Food and Drug Administration, created under Republic Act No. 3720, series of 1963, as amended by Executive Order 175, series of 1987, otherwise known as the “Food, Drugs and Devices, and Cosmetics Act”, and subsequently Republic Act No. 9711 otherwise known as “The Food and Drug Administration Act of 2009”, is mandated to ensure the safety, efficacy or quality of health products as defined by RA No. 97111, which include means food, drugs, cosmetics, devices, biologicals, vaccines, in-vitro diagnostic reagents, radiation-emitting devices or equipment, and household/urban hazardous substances, including pesticides and toys, or consumer products that may have an effect on health which require regulations as determined by the FDA.

Functions of FDA
In order to protect and promote the right to health of the Filipino people and to establish and maintain an effective health products regulatory system responsive to the country’s health needs and problems, the FDA shall perform the following functions as provided by existing laws:
** To establish safety or efficacy standards and quality measures for foods, drugs and devices and cosmetics and other health product;
** To undertake appropriate health manpower development and research, responsive to the country’s health needs and problems;
** To assume primary jurisdiction in the collection of samples of health products;
** To analyze and inspect health products;
** To establish analytical data to serve as basis for the preparation of health products standards, and to recommend standards of identity, purity, safety, efficacy, quality and fill of container;
** To issue certificates of compliance with technical requirements to serve as basis for the issuance of appropriate authorization and spot-check for compliance with regulations regarding operation of manufacturers, importers, exporters, distributors, wholesalers, drug outlets, and other establishments and facilities of health products, as determined by the FDA
** To conduct appropriate tests on all applicable health products prior to the issuance of appropriate authorizations to ensure safety, efficacy, purity, and quality;
** To require all manufacturers, traders, distributors, importers, exporters, wholesalers, retailers, consumers, and non-consumer users of health products to report to the FDA any incident that reasonably indicates that said product has caused or contributed to the death, serious illness or serious injury to a consumer, a patient, or any person;
** To issue cease and desist orders motu propio or upon verified complaint for health produts, whether or not registered with the FDA Provided, That for registered health products, the cease and desist order is valid for thirty (30) days and may be extended for sixty (60) days only after due process has been observed;
** To order the ban, recall, and/or withdrawal of any health product, after due process, found to have caused the death, serious illness or serious injury to a consumer or patient, or is found to be imminently injurious, unsafe, dangerous, or grossly deceptive, and to require all concerned to implement the risk management plan which is a requirement for the issuance of the appropriate authorization;
** To strengthen the post market surveillance system in monitoring health products as defined in this Act and incidents of adverse events involving such products;
** To develop and issue standards and appropriate authorizations that would cover establishments, facilities and health products;
** To conduct, supervise, monitor and audit research studies on health and safety issues of health products undertaken by entities duly approved by the FDA;
** To prescribe standards, guidelines, and regulations with respect to information, advertisements and other marketing instruments and promotion, sponsorship, and other marketing activities about the health products
** To maintain bonded warehouses and/or establish the same, whenever necessary or appropriate, as determined by the director-general for confiscated goods in strategic areas of the country especially at major ports of entry; and
** To exercise such other powers and perform such other functions as may be necessary to carry out its duties and responsibilities.
View Comments (10)
Good day.
Please update us on the status of our Letter of Request for Clarification. Document Tracking No. 20191105150127.
I believe the response to this request could be available in 15 working days as they promised response in 10 workings.
Please advise on what we can do to secure the clarification on AO 2019 0019 for out product Vigormin for wastewater treatment,
Thank you.
Hi, Til now I have not yet received my LTO since I submit at Fda Iloilo last jan.14,2019 my document tracking number 20190114122113
Til now, I have not yet received my LTO, since I submit at FDA La Union last December 27th, 2018. Please reply.
I have entered my DTL tracking ID 20171213141641 and my email address. After submit, the return message is "No route to host" Why? I want to know the status of my CPR because I am going to use it.
We already sent our complete documents/payments for LTO renewal of our X-ray Facility here in Cagayan de Oro City, FDA since last October 26, 2016 then until now we didn't receive any feedback about the status of our documents. This is from Zendrug, Inc., Suan Arcade,Ilaya Carmen, Cagayan de Oro City. Hope we hear a response soon.
Where will I get the track number?
How many days required to receive your track no.? Do we need it before we pay the fees?
It has been more than 4 months already and our LTO copy hasn't arrived yet and I would like to track the doc status but I wasn't given any track no. How can we get it?
Why isn't any status or date shown after I input the tracking number and phone number? All I see is Date and status. The rest is blank
Right. Literally we will see the word "status" and their is blank. Very inefficient feature,